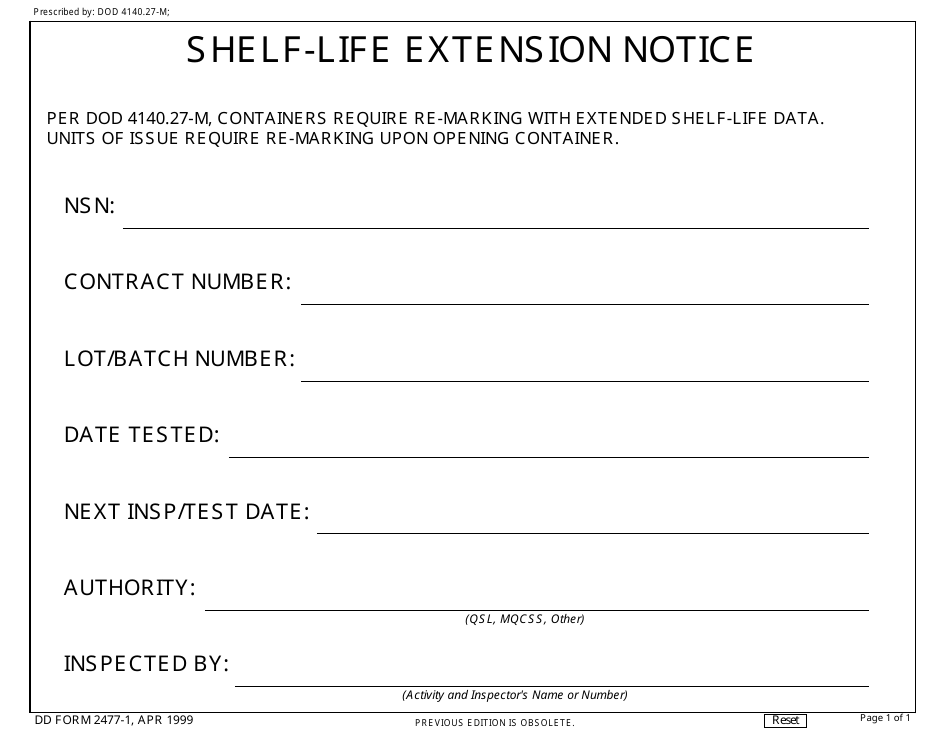
Shelf Life Extension Plan Impd . Guideline on the requirements for quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. biological product, investigational medicinal product (imp), clinical trial, quality.
from www.templateroller.com
In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. Guideline on the requirements for quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. biological product, investigational medicinal product (imp), clinical trial, quality.
DD Form 24771 Fill Out, Sign Online and Download Fillable PDF
Shelf Life Extension Plan Impd biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. biological product, investigational medicinal product (imp), clinical trial, quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. Guideline on the requirements for quality.
From www.researchgate.net
Shelflife extension of whole fruits and vegetables at (a) room Shelf Life Extension Plan Impd For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. biological product, investigational medicinal product (imp), clinical trial, quality. Guideline on the requirements for. Shelf Life Extension Plan Impd.
From www.slideshare.net
Guide to DuPont™ Danisco® shelf life extension solutions Shelf Life Extension Plan Impd biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. Guideline on the requirements for quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.
From www.templateroller.com
DD Form 24771 Fill Out, Sign Online and Download Fillable PDF Shelf Life Extension Plan Impd biological product, investigational medicinal product (imp), clinical trial, quality. Guideline on the requirements for quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference. Shelf Life Extension Plan Impd.
From www.templateroller.com
DD Form 24771 Download Fillable PDF or Fill Online ShelfLife Shelf Life Extension Plan Impd Guideline on the requirements for quality. biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.
From www.slideserve.com
PPT Shelf Life Extension Program (SLEP) PowerPoint Presentation, free Shelf Life Extension Plan Impd Guideline on the requirements for quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference. Shelf Life Extension Plan Impd.
From www.galdi.it
How to Extend the Shelf Life of Food Products Galdi Shelf Life Extension Plan Impd biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. Guideline on the requirements for. Shelf Life Extension Plan Impd.
From www.snapdeal.com
Preservation and Shelf Life Extension Buy Preservation and Shelf Life Shelf Life Extension Plan Impd In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. biological product, investigational medicinal product (imp), clinical trial, quality. Guideline on the requirements for. Shelf Life Extension Plan Impd.
From www.lbs-biotech.com
Extension of Shelf Life LBS Shelf Life Extension Plan Impd In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. biological product, investigational medicinal product (imp), clinical trial, quality. Guideline on the requirements for quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference. Shelf Life Extension Plan Impd.
From www.academia.edu
(PDF) Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Shelf Life Extension Plan Impd biological product, investigational medicinal product (imp), clinical trial, quality. Guideline on the requirements for quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.
From www.scommerce-mage.com
MicroManage Your Inventory with Magento 2 Product Shelf Life Extension Shelf Life Extension Plan Impd Guideline on the requirements for quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. biological product, investigational medicinal product (imp), clinical trial,. Shelf Life Extension Plan Impd.
From www.youtube.com
Shelf Life Extension For Sterile Medical Devices STERIS AST TechTalk Shelf Life Extension Plan Impd Guideline on the requirements for quality. biological product, investigational medicinal product (imp), clinical trial, quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference. Shelf Life Extension Plan Impd.
From onetray.com
ONE TRAY® Extended Shelf Life Testing ONE TRAY® Shelf Life Extension Plan Impd Guideline on the requirements for quality. biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.
From www.semanticscholar.org
Figure 1 from ShelfLife Extension of Wood Apple Beverages Maintaining Shelf Life Extension Plan Impd Guideline on the requirements for quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. biological product, investigational medicinal product (imp), clinical trial,. Shelf Life Extension Plan Impd.
From www.scommerce-mage.com
MicroManage Your Inventory with Magento 2 Product Shelf Life Extension Shelf Life Extension Plan Impd biological product, investigational medicinal product (imp), clinical trial, quality. Guideline on the requirements for quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.
From www.dobbsferry.com
IHEALTH COVID19 TEST KIT SHELF LIFE EXTENSION INFORMATION Village of Shelf Life Extension Plan Impd For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. Guideline on the requirements for quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. biological product, investigational medicinal product (imp), clinical trial,. Shelf Life Extension Plan Impd.
From www.rightwayfoodservice.com
5 Shelf Life Extension Plan Impd Guideline on the requirements for quality. biological product, investigational medicinal product (imp), clinical trial, quality. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires in. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference. Shelf Life Extension Plan Impd.
From www.aplyon.com
Shelf Life Procedure Shelf Life Extension Plan Impd Guideline on the requirements for quality. biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.
From www.slideshare.net
Guide to DuPont™ Danisco® shelf life extension solutions Shelf Life Extension Plan Impd Guideline on the requirements for quality. biological product, investigational medicinal product (imp), clinical trial, quality. For drug substances or imps to be used in clinical trials as described in chapters 2 to 8, reference to. In general, the extension of the rp for the ds or of the sl for the imp in support of a clinical study requires. Shelf Life Extension Plan Impd.